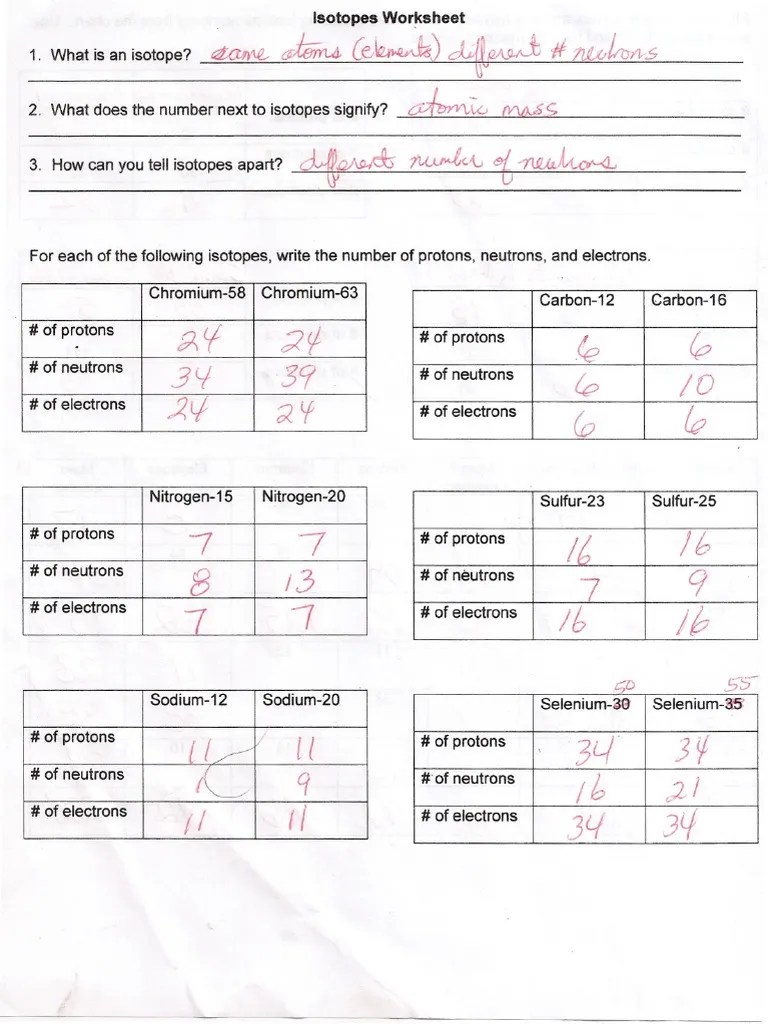
Isotopes Worksheet
Isotopes Worksheet - Free interactive exercises to practice online or download as pdf to print. The simplest example of an atom with different isotopes is hydrogen. How can you tell isotopes of the same element apart? As mentioned in the previous section, atoms that have the same atomic number (number of protons), but different mass numbers (number of protons and neutrons) are called isotopes (nuclides). I can use the atomic number of an element to identify protons and electrons for any element. The number 6 refers to the atomic number c. The number of protons in an atom determines the identity of the atom. The numbers 12, 13, and 14 refer to the mass number d. I can apply the relationship, “mass number = protons + neutrons,” to find protons, neutrons, or mass for any element Protons and neutrons both have a mass of 1 amu.
As mentioned in the previous section, atoms that have the same atomic number (number of protons), but different mass numbers (number of protons and neutrons) are called isotopes (nuclides). Describe the general arrangement of subatomic particles in the atom. Use the periodic table to identify and count subatomic particles within the atom. What does the number next to isotopes signify? Isotopes are versions of the same element. Fill in the table with the correct information. How can you tell isotopes apart?
What does the number next to isotopes signify? As mentioned in the previous section, atoms that have the same atomic number (number of protons), but different mass numbers (number of protons and neutrons) are called isotopes (nuclides). Describe the general arrangement of subatomic particles in the atom. How can you tell isotopes of the same element apart? What contribution did these scientists make to atomic models of the atom?
Isotopes Worksheet Answers
12c 6 13c 14c 6 6 a. I can apply the relationship, “mass number = protons + neutrons,” to find protons, neutrons, or mass for any element The numbers 12, 13, and 14 refer to the mass number d. Web here are three isotopes of an element: There are naturally occurring isotopes and isotopes that are artificially produced.
Isotopes Worksheet 1 Answer Key kidsworksheetfun
The number indicates the isotope’s mass number. # protons = # electrons. Web isotope practice worksheet atoms of a given element which have the same number of protons but different numbers of neutrons are called isotopes. 12c 6 13c 14c 6 6 a. Fill in the table with the correct information.
Isotopes Worksheet 1 Worksheet for 9th 12th Grade Lesson
The numbers 12, 13, and 14 refer to the mass number d. Web atoms and isotopes worksheet 1. I can use the atomic number of an element to identify protons and electrons for any element. What does the number next to isotopes signify? As mentioned in the previous section, atoms that have the same atomic number (number of protons), but.
Isotope Practice Worksheet
What does the number next to isotopes signify? As mentioned in the previous section, atoms that have the same atomic number (number of protons), but different mass numbers (number of protons and neutrons) are called isotopes (nuclides). There are naturally occurring isotopes and isotopes that are artificially produced. What contribution did these scientists make to atomic models of the atom?.
5 Isotope Practice Worksheet
How can you tell isotopes of the same element apart? Isotopes are versions of the same element. The number 6 refers to the atomic number c. What does the number next to isotopes signify? Web isotope practice worksheet name:
Isotopes Ions And Atoms Worksheet 1 Answer Key —
The numbers 12, 13, and 14 refer to the mass number d. I can use the atomic number of an element to identify protons and electrons for any element. 12c 6 13c 14c 6 6 a. Web here are three isotopes of an element: The simplest example of an atom with different isotopes is hydrogen.
Unit 2 Chapters 4, 5 & 6 Mrs. Gingras' Chemistry Page
Use the periodic table to identify and count subatomic particles within the atom. How many protons and neutrons are in the second isotope? In a neutral atom, the number of positive protons equals the number of negative electrons. The simplest example of an atom with different isotopes is hydrogen. There are naturally occurring isotopes and isotopes that are artificially produced.
50 Isotopes Worksheet Answer Key
# protons = # electrons. _____ _____ for each of the following isotopes, write the # of protons, neutrons, and electrons. I can apply the relationship, “mass number = protons + neutrons,” to find protons, neutrons, or mass for any element Protons and neutrons both have a mass of 1 amu. Web isotope practice worksheet 1.
Most Common Isotope Worksheet 1
How can you tell isotopes of the same element apart? Worksheets for a lesson on isotopes, including a full set of answers on powerpoint slides. What does the number next to isotopes signify? Use the periodic table to identify and count subatomic particles within the atom. They have the same number of protons and electrons as the element but different.
Isotopes Worksheet - Web isotope practice worksheet name: Web atoms and isotopes worksheet 1. I can use the atomic number of an element to identify protons and electrons for any element. Protons and neutrons both have a mass of 1 amu. The numbers 12, 13, and 14 refer to the mass number d. The number of protons in an atom determines the identity of the atom. # protons = # electrons. The number 6 refers to the atomic number c. Thus, isotopes have the same position in the periodic table, the same chemical properties and the same atomic charge. In a neutral atom, the number of positive protons equals the number of negative electrons.
_____ _____ for each of the following isotopes, write the # of protons, neutrons, and electrons. Protons and neutrons both have a mass of 1 amu. Free interactive exercises to practice online or download as pdf to print. As mentioned in the previous section, atoms that have the same atomic number (number of protons), but different mass numbers (number of protons and neutrons) are called isotopes (nuclides). What contribution did these scientists make to atomic models of the atom?
What does the number next to isotopes signify? Web isotope practice worksheet 1. How many protons and neutrons are in the first isotope? Use the periodic table to identify and count subatomic particles within the atom.
The Number Indicates The Isotope’s Mass Number.
How can you tell isotopes of the same element apart? Fill in the table with the correct information. What does the number next to isotopes signify? Web here are three isotopes of an element:
Isotopes Are Versions Of The Same Element.
There are naturally occurring isotopes and isotopes that are artificially produced. Free interactive exercises to practice online or download as pdf to print. What does the number next to isotopes signify? I can use the atomic number of an element to identify protons and electrons for any element.
How Many Protons And Neutrons Are In The Second Isotope?
Web atoms and isotopes worksheet 1. Atomic # = # protons. The lesson on “atomic structure and notation” is provided free in my other resources, so you can try before you buy. What contribution did these scientists make to atomic models of the atom?
Describe The General Arrangement Of Subatomic Particles In The Atom.
In a neutral atom, the number of positive protons equals the number of negative electrons. The number 6 refers to the atomic number c. Use the periodic table to identify and count subatomic particles within the atom. Worksheets for a lesson on isotopes, including a full set of answers on powerpoint slides.