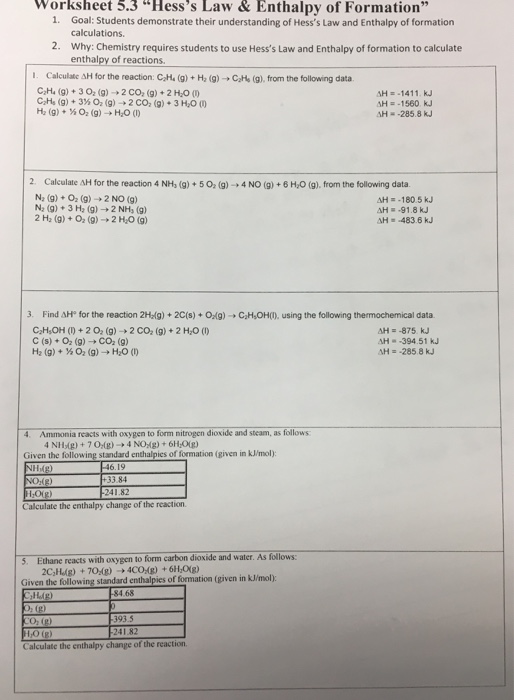
Hess's Law Worksheet
Hess's Law Worksheet - Web hess's law worksheet calculate h for the reaction: 3c (s) + 4h2 (g) → c3h8(g) δh f (propane) can’t be found. Web hess's law worksheet ‐ answers calculate ∆h for the reaction: _____ student id#:_____ work in groups on these problems. Calculate h for the reaction: Hess’s law lesson menu lesson lesson plan lesson presentation lesson video lesson explainer lesson playlist lesson worksheet course menu chemistry • first. C2h4 (g) + h2 (g) → c2h6 (g), from the following data. If the new window covers up. Web know the first law of thermodynamics. Web chemical energy hess's law.
So, if 100 units of energy are involved when p forms q i.e. Web chemical energy hess's law. When you press new problem a window will appear which presents a hess' law scenario. Calculate h for the reaction: C2h4 (g) + 3 o2 (g) → 2 co2 (g) + 2 h2o (l) c2h6. Web hess's law of constant heat summation (or just hess's law) states that regardless of the multiple stages or steps of a reaction, the total enthalpy change for the. Web heat of formation worksheet.
Web the hess law affirms that x equals the sum of y and z. C2h4 (g) + h2 (g) → c2h6 (g), from the following data. Web hess’s law can be stated as ‘the heat evolved or absorbed in a chemical process is the same, whether the process takes place in one or in several steps’. Web hess’ law is used to calculate enthalpy changes which can’t be found experimentally using calorimetry, eg: Web know the first law of thermodynamics.
Solved worksheet 5.3 “ Hess 's Law & Enthalpy of Formation "
Web hess law worksheet uploaded by natsmd chem copyright: Web heat of formation worksheet. Use a standard enthalpies of formation table to determine the change in enthalpy for each of these reactions. Web hess's law worksheet calculate h for the reaction: Web the hess law affirms that x equals the sum of y and z.
️Enthalpy Of Formation Worksheet Free Download Goodimg.co
Web hess's law (worksheet) page id. Use a standard enthalpies of formation table to determine the change in enthalpy for each of these reactions. Web hess law worksheet uploaded by natsmd chem copyright: Web hess’ law is used to calculate enthalpy changes which can’t be found experimentally using calorimetry, eg: Web hess's law worksheet ‐ answers calculate ∆h for the.
WS_Hess`s Law
Web hess' law this page is an exercise in using hess' law. Understand the relationships between heat, work, internal energy, and enthalpy. C2h4 (g) + h2 (g) → c2h6 (g), from the following data. Web hess's law worksheet ‐ answers calculate ∆h for the reaction: Web know the first law of thermodynamics.
Hess' Law Worksheet 4/15
Web hess's law worksheet ‐ answers calculate ∆h for the reaction: If the new window covers up. Understand the concepts of heat. Web hess's law worksheet calculate h for the reaction: Web doc, 45 kb docx, 197.01 kb a powerpoint and accompanying worksheets for an as chemistry lesson on hess's law, following the aqa spec.
Solved WORKSHEET HESS'S LAW NAME 1) Use Hess's law to
Web hess's law states that if a process can be expressed as the sum of two or more steps, the enthalpy change for the overall process is the sum of the δh values for each step. Web hess' law this page is an exercise in using hess' law. So, if 100 units of energy are involved when p forms q.
Worksheet 1
Web doc, 45 kb docx, 197.01 kb a powerpoint and accompanying worksheets for an as chemistry lesson on hess's law, following the aqa spec. Web hess's law worksheet ‐ answers calculate ∆h for the reaction: X = 100 then, (y+z) will also equal 100 units. Web hess's law states that if a process can be expressed as the sum of.
Hess S Law Worksheet Answer Key Thekidsworksheet
Web doc, 45 kb docx, 197.01 kb a powerpoint and accompanying worksheets for an as chemistry lesson on hess's law, following the aqa spec. Web hess’s law can be stated as ‘the heat evolved or absorbed in a chemical process is the same, whether the process takes place in one or in several steps’. C2h4 (g) + 3 o2 (g).
Hess Law Worksheet Answers
3c (s) + 4h2 (g) → c3h8(g) δh f (propane) can’t be found. Web hess's law worksheet ‐ answers calculate ∆h for the reaction: Web hess' law this page is an exercise in using hess' law. Web hess’s law can be stated as ‘the heat evolved or absorbed in a chemical process is the same, whether the process takes place.
Hess Law Worksheet Pdf Thekidsworksheet
Web doc, 45 kb docx, 197.01 kb a powerpoint and accompanying worksheets for an as chemistry lesson on hess's law, following the aqa spec. When you press new problem a window will appear which presents a hess' law scenario. Web hess’ law is used to calculate enthalpy changes which can’t be found experimentally using calorimetry, eg: So, if 100 units.
Hess's Law Worksheet - Web know the first law of thermodynamics. Web heat of formation worksheet. Calculate h for the reaction: Understand the concepts of heat. Hess’s law lesson menu lesson lesson plan lesson presentation lesson video lesson explainer lesson playlist lesson worksheet course menu chemistry • first. So, if 100 units of energy are involved when p forms q i.e. Web in short, the hess’s law is based on the fact that enthalpy is a state function, and therefore, δh rxn is the same whether the reaction takes place in one step or in a series of steps. C2h4 (g) + h2 (g) → c2h6 (g), from the following data. I can&'t take credit for. When you press new problem a window will appear which presents a hess' law scenario.
Calculate h for the reaction: When you press new problem a window will appear which presents a hess' law scenario. Web know the first law of thermodynamics. Web heat of formation worksheet. Web hess's law worksheet ‐ answers calculate ∆h for the reaction:
So, if 100 units of energy are involved when p forms q i.e. Understand the concepts of heat. I can&'t take credit for. If the new window covers up.
Understand The Concepts Of Heat.
Web chemical energy hess's law. Web hess' law this page is an exercise in using hess' law. Web doc, 45 kb docx, 197.01 kb a powerpoint and accompanying worksheets for an as chemistry lesson on hess's law, following the aqa spec. Web hess's law worksheet ‐ answers calculate ∆h for the reaction:
Energy Changes Occur In Chemical Reactions As Bonds Are Broken And New Bonds Formed.
X = 100 then, (y+z) will also equal 100 units. Web heat of formation worksheet. I can&'t take credit for. Web hess’ law is used to calculate enthalpy changes which can’t be found experimentally using calorimetry, eg:
C2H4 (G) + 3 O2 (G) → 2 Co2 (G) + 2 H2O (L) C2H6.
Web hess's law (worksheet) page id. Use a standard enthalpies of formation table to determine the change in enthalpy for each of these reactions. Web the hess law affirms that x equals the sum of y and z. Web know the first law of thermodynamics.
C2H4 (G) + H2 (G) → C2H6 (G), From The Following Data.
Web hess's law states that if a process can be expressed as the sum of two or more steps, the enthalpy change for the overall process is the sum of the δh values for each step. 3c (s) + 4h2 (g) → c3h8(g) δh f (propane) can’t be found. Web in short, the hess’s law is based on the fact that enthalpy is a state function, and therefore, δh rxn is the same whether the reaction takes place in one step or in a series of steps. If the new window covers up.